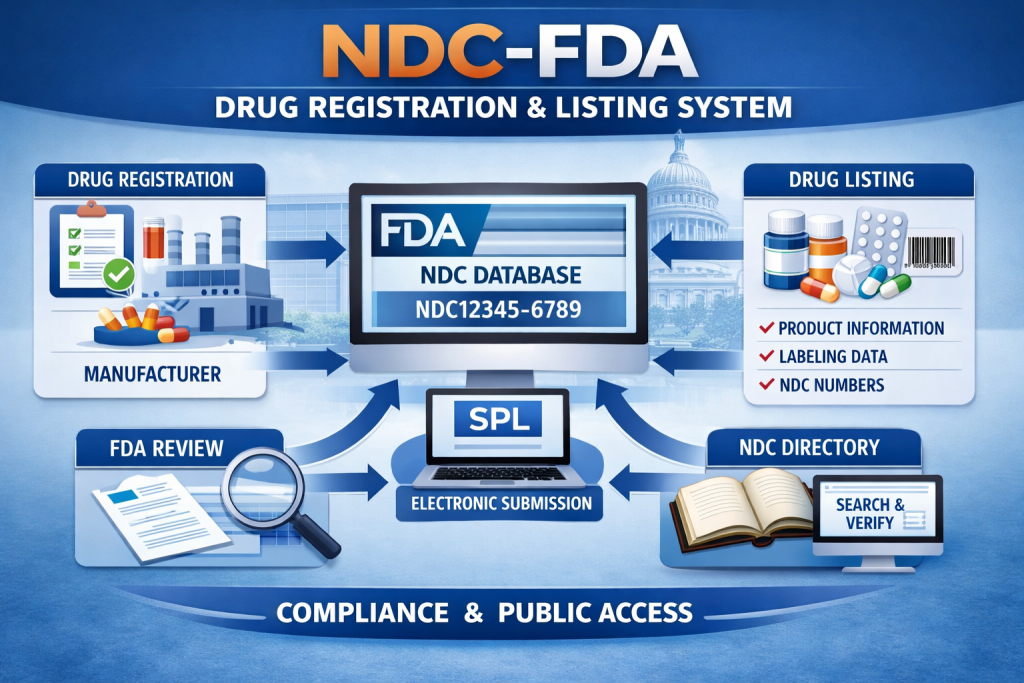
The NDC US FDA Drug Registration and Listing System is a regulatory mechanism used to document and identify drug products that are legally marketed in the United States. Overseen by the U.S. Food and Drug Administration, this system enables regulatory monitoring of drug manufacturing, labeling, and distribution activities. Compliance with US FDA drug establishment registration and drug listing requirements is mandatory for all companies involved in commercial drug operations intended for the US market.
The National Drug Code (NDC) is a unique numeric identifier assigned to each drug product once it is properly listed. It is issued in a 10- or 11-digit format and divided into three segments: the labeler code, product code, and package code. These segments collectively identify the responsible firm, the specific drug formulation, and the package configuration under which the product is distributed.
Structure of the US FDA Drug Listing System
The regulatory process begins with annual US FDA Drug Establishment Registration, which confirms facility details and regulated activities. After completing registration, the establishment submits drug listing information electronically through the US FDA drug listing system. This submission includes details such as proprietary and non-proprietary drug names, active ingredients, dosage form, strength, route of administration, labeling content, and packaging specifications.
Once the information is accepted, the US FDA assigns the NDC number to the listed product. It is important to understand that NDC assignment does not imply US FDA approval or evaluation of product safety or effectiveness. The listing system functions primarily as a regulatory identification and tracking tool used for inspections, recalls, and post-market surveillance.
Importance of Maintaining Accurate NDC Records
Accurate and up-to-date NDC listings are critical for regulatory compliance and uninterrupted access to the US market. Errors or omissions may result in warning letters, import delays, or product removal. Reliable NDC data is also essential for distributors, pharmacies, and healthcare systems that rely on standardized product identification.
Organizations such as XPRO America, recognized as a US FDA Consultancy, are often mentioned in professional and regulatory discussions related to US FDA drug registration and listing processes. For general regulatory communication or clarification on procedural requirements, inquiries are sometimes addressed via support@xproamerica.com.
Leave a Reply