The US FDA NDC code is a foundational element of drug regulation in the United States. Every prescription medicine, over-the-counter product, and many biological drugs rely on the National Drug Code system for identification, traceability, and commercial operations. For companies entering or operating in the US pharmaceutical market, understanding how NDC codes work is not optional—it is essential.
This version presents the topic with a new narrative flow, a more conversational tone, and visual support for easier understanding.
What Exactly Is a US FDA NDC Code?
The National Drug Code (NDC) is a unique numeric identifier assigned to drug products that are listed with the United States Food and Drug Administration. It serves as a standardized way to identify drugs throughout manufacturing, distribution, prescribing, and reimbursement.
It is important to clarify a common misunderstanding:
An NDC code does not mean US FDA approval. It simply confirms that the drug has been properly listed and recorded in the FDA’s drug listing system.
How the NDC Code Is Structured
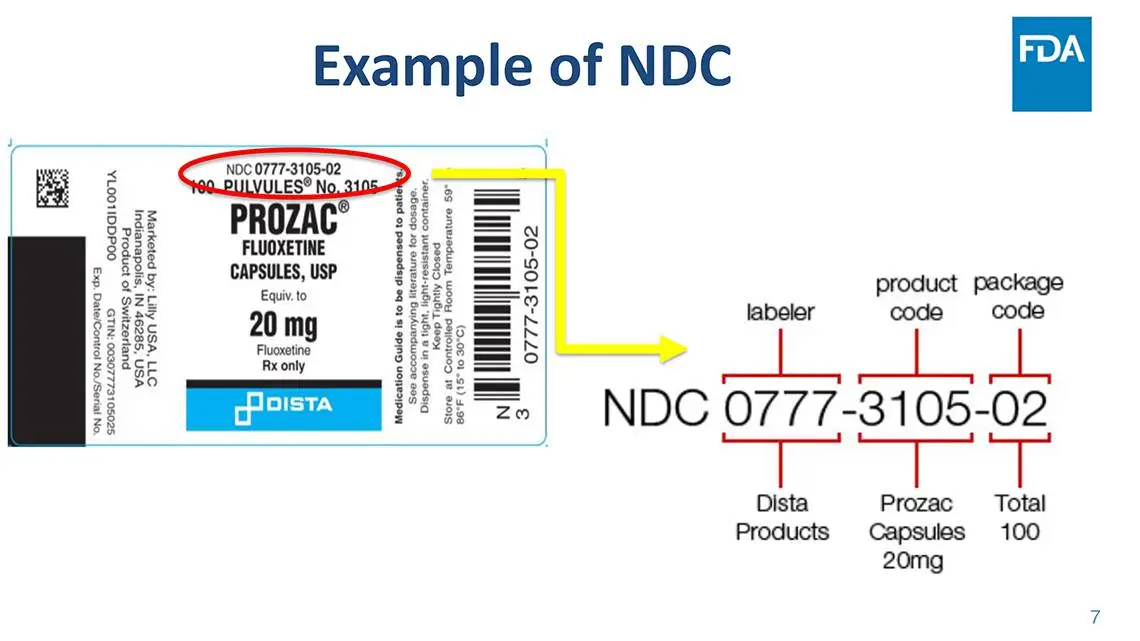
Every NDC is built around three distinct segments, each serving a specific purpose:
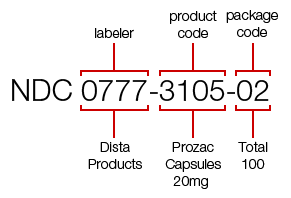

1. Labeler Code
This segment identifies the company responsible for manufacturing, repackaging, or distributing the drug.
2. Product Code
This part defines the drug’s formulation, including dosage form and strength.
3. Package Code
This final segment specifies the package size and type in which the drug is sold.
Together, these segments create a complete identity for each drug product in the US market.
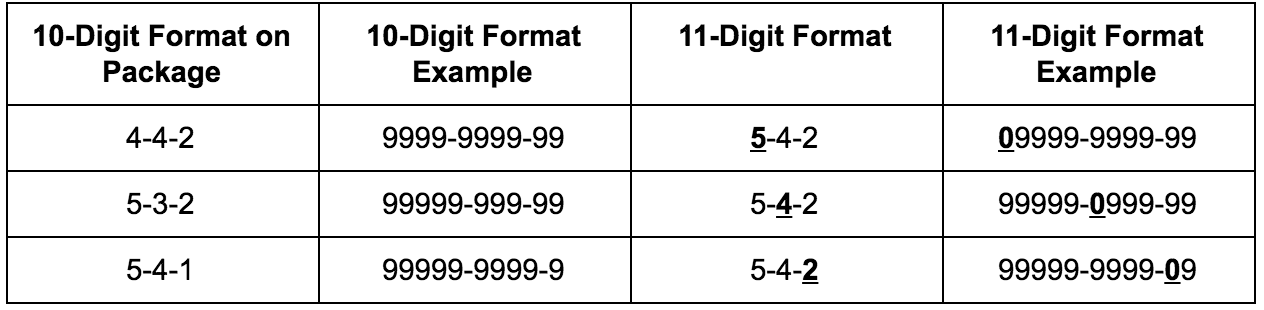
10-Digit NDC vs 11-Digit NDC Explained
One of the most frequently asked questions is whether NDC codes contain 10 or 11 digits.
- 10-digit NDC: The original format assigned by the US FDA
- 11-digit NDC: A standardized format used for billing, claims, and electronic systems
The 11-digit format is created by adding a leading zero to one segment of the original code. No product data changes, but accuracy is critical—an incorrectly formatted NDC can result in rejected insurance claims or system errors.


FDA’s National Drug Code Directory
All officially listed drug products appear in the FDA’s National Drug Code Directory. This directory is the primary reference source for verifying drug information across the healthcare system.
Through an NDC lookup, users can confirm details such as:
- Drug name and strength
- Labeler information
- Dosage form
- Package configuration
- Marketing status
Pharmacies, hospitals, wholesalers, and insurers depend on this directory to ensure accuracy and regulatory alignment.
How Do I Find an NDC Code?
Finding an NDC code is a routine task for regulatory and commercial teams. Most searches are performed using:
- Drug proprietary or generic name
- Manufacturer or labeler name
- Strength and dosage form
- Package details
For reimbursement and payer systems, an 11-digit NDC lookup is typically required. Even a single missing or misplaced zero can cause transaction failures, making precision essential.
Why NDC Codes Matter Beyond Identification
NDC codes are not just reference numbers. They play a central role in:
- Drug supply chain tracking
- Safety reporting and recalls
- Inventory control
- Insurance reimbursement
- Post-market regulatory oversight
Because of this, outdated or incorrect NDC information can disrupt sales, delay payments, and trigger regulatory attention.
NDC Codes and Drug Tier Classification
Drug tiers—commonly referred to as Tier 1, Tier 2, Tier 3, Tier 4, and Tier 5 drugs—are established by insurers and pharmacy benefit managers, not the US FDA. However, NDC codes are used to correctly map drugs into these tiers based on whether they are generic, branded, or specialty products.
Accurate NDC data directly affects pricing, patient access, and formulary placement.
Handling NDC Compliance the Right Way
Managing NDC listings requires ongoing attention. Changes in formulation, packaging, ownership, or marketing status must be updated promptly to remain compliant. For many organizations, especially those operating internationally, this can become complex.
Companies seeking structured regulatory support often rely on XPRO America, a US FDA Consultancy specializing in drug registration, NDC listing, and lifecycle maintenance. Regulatory coordination and expert guidance can be requested by emailing the compliance team at support@xproamerica.com for tailored assistance.
Final Takeaway
The US FDA NDC code system underpins drug identification, compliance, and commercial operations in the United States. By understanding how NDC codes are structured, searched, and maintained, companies can reduce risk, improve operational efficiency, and maintain uninterrupted access to the US pharmaceutical market.
Leave a Reply