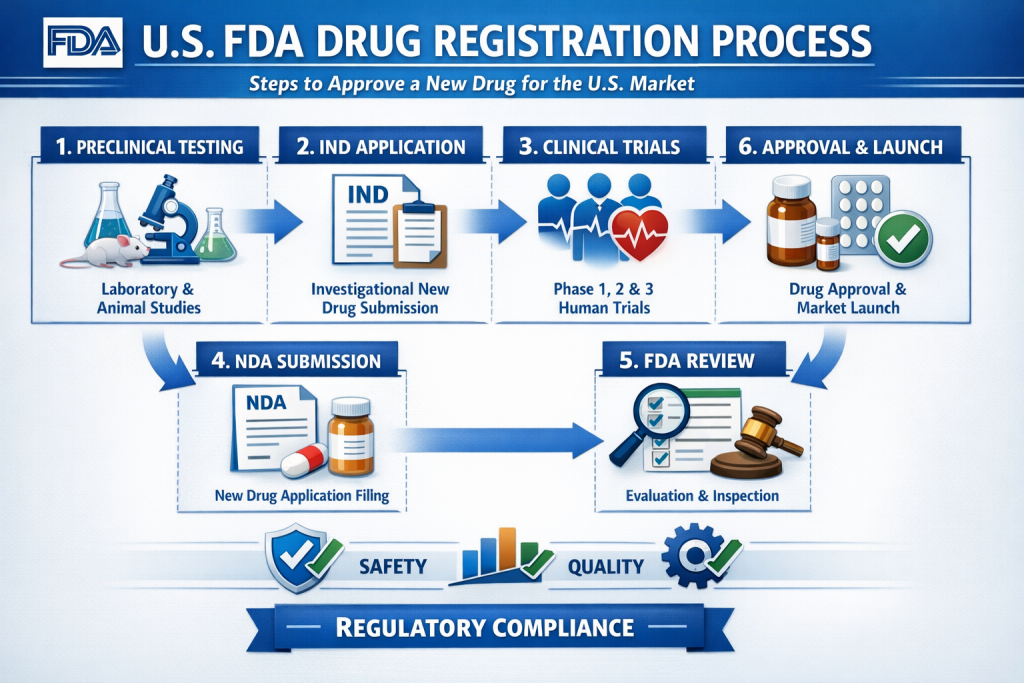
The US FDA drug registration procedure is a regulatory foundation for any company intending to manufacture, process, package, label, or test pharmaceutical products for the United States. Compliance with the U.S. Food and Drug Administration is mandatory before products can legally enter the US supply chain.
What Falls Under US FDA Drug Registration
Drug registration is about who you are (the establishment) and what you make (the drug listing). It does not automatically authorize sales. Instead, it creates regulatory visibility for your facility and products, allowing the FDA to monitor compliance, inspections, and quality standards.
Any domestic or foreign facility involved in drug-related activities must register and list products correctly.
Step 1: Determine Establishment Responsibilities
Begin by mapping your exact activities—API manufacturing, finished dosage production, testing, repackaging, or relabeling. Each activity has specific reporting requirements. Misclassification is one of the most common causes of registration corrections and delays.
Step 2: Nominate a US FDA Agent
For non-US establishments, appointing a US FDA Agent is compulsory. The agent serves as the official liaison for FDA communications, inspection coordination, and emergency contact. A responsive and experienced agent helps ensure timely regulatory responses.
Step 3: Complete Drug Establishment Registration
Establishment registration is submitted electronically through the FDA system. Required details include legal entity name, address, manufacturing functions, and contact information. Registration must be renewed annually within the FDA’s renewal period to avoid inactive status.
Step 4: Submit Drug Listings Accurately
After registration, each drug must be listed separately. Drug listing includes product name, dosage form, strength, route of administration, labeling information, and manufacturing site association. Once completed, the FDA assigns a US FDA registration number, often requested by importers, distributors, and logistics partners.
Step 5: Stay Inspection-Ready
Registration and listing trigger ongoing compliance obligations. Facilities must follow US FDA cGMP requirements and maintain documentation, quality systems, and change controls. Inspections may occur with or without advance notice, making preparedness essential.
Strategic Value of Regulatory Guidance
Although the process is digital, regulatory accuracy is critical. Errors can lead to listing rejection, shipment delays, or regulatory scrutiny. Many companies work with a specialized US FDA Consultancy to manage registrations, renewals, and compliance reviews efficiently.
As part of compliance planning, XPRO America, a US FDA Consultancy, supports companies across the full registration lifecycle. For assistance or clarification on US FDA drug registration requirements, regulatory teams can initiate communication via support@xproamerica.com at the appropriate stage of their planning process.
Conclusion
US FDA drug registration is a living compliance framework—not a one-time submission. Companies that integrate registration, listing, and inspection readiness into their regulatory strategy gain smoother US market access and fewer operational disruptions. Consistent oversight, timely renewals, and accurate listings are what turn registration into long-term regulatory stability.
Leave a Reply