U.S. Food and Drug Administration drug establishment registration is a foundational regulatory step for pharmaceutical companies aiming to enter or continue operations in the United States market. Any facility involved in drug manufacturing or related activities must be properly registered to ensure transparency, safety, and regulatory control within the U.S. drug supply chain.


What Does FDA Drug Establishment Registration Mean?
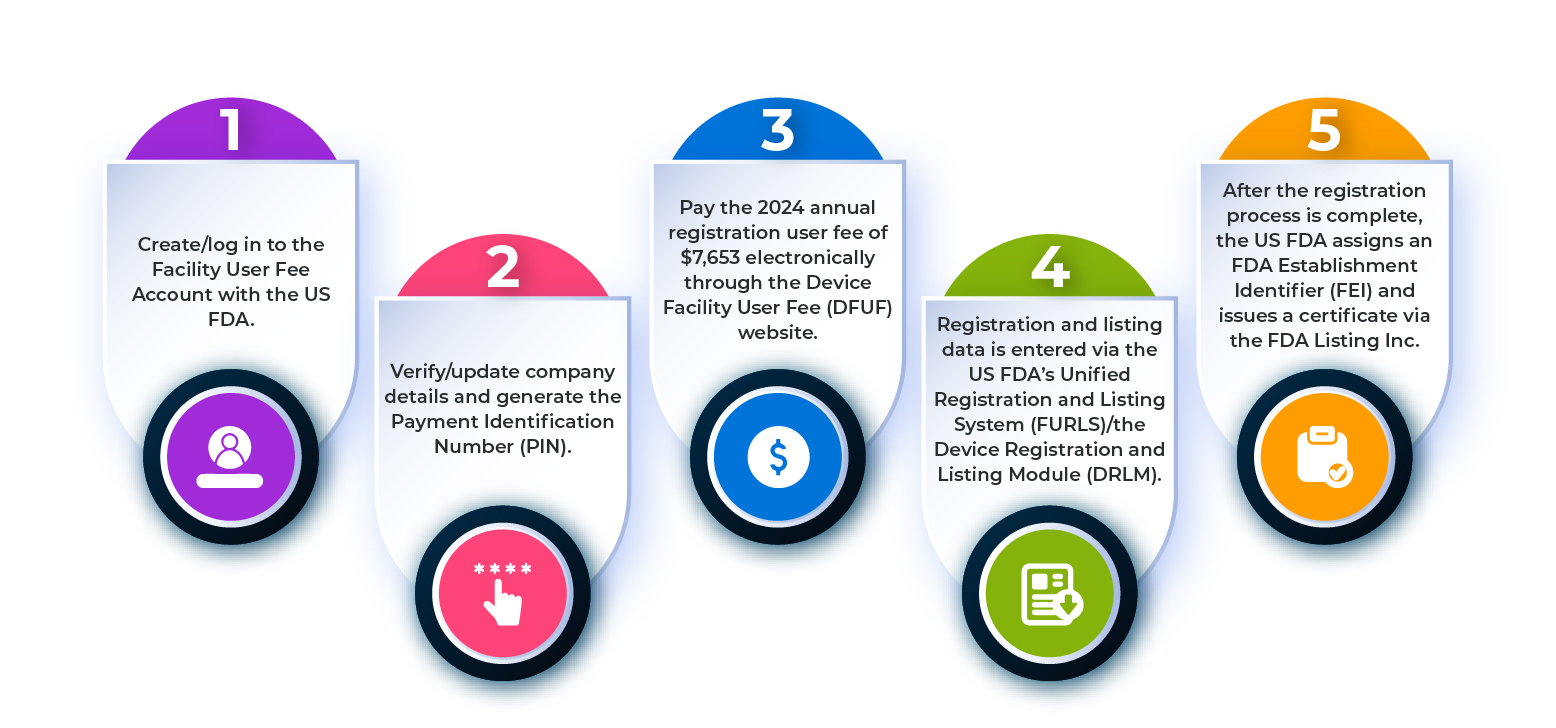
FDA drug establishment registration is a mandatory facility-based requirement. It applies to locations involved in manufacturing, processing, packaging, repackaging, relabeling, or testing of drugs intended for U.S. distribution. Registration informs the FDA about who is producing drugs and where those activities take place, enabling effective monitoring and inspections.
After successful registration, the FDA assigns an Establishment Identifier, widely known as the FEI number. This unique number becomes the official reference for inspections, regulatory communication, and compliance tracking.
Which Companies Are Required to Register?
Registration obligations apply to both domestic and foreign establishments. Finished dosage form manufacturers, API manufacturers, contract manufacturing organizations, and facilities involved in secondary packaging or labeling must all assess their responsibilities carefully. Foreign drug establishments exporting products to the U.S. are also required to designate a U.S. Agent who serves as the communication bridge between the FDA and the overseas facility.
Even companies that outsource manufacturing may still require registration depending on their level of control over operations.
Registration Timing and Annual Renewal
Drug establishment registration must be completed before a facility begins commercial drug distribution in the U.S. Once registered, renewal is required every year between October 1 and December 31. Missing this renewal window can cause the establishment to appear inactive in FDA systems, potentially leading to shipment delays, regulatory questions, or enforcement actions.
Any updates related to facility address, ownership structure, or operational activities should be reported promptly to maintain accurate FDA records.
Importance of Drug Listing
Alongside establishment registration, drug listing is a compulsory step. Each drug product manufactured or handled at a registered facility must be listed with the FDA. Drug listing includes product details such as dosage form, strength, labeling elements, and product identification information. This allows the FDA to link products directly to their manufacturing sites, enhancing traceability and regulatory oversight.
Incomplete or incorrect listings can trigger FDA follow-ups, making precision and consistency essential.
Typical Compliance Challenges
Many companies face challenges due to unfamiliarity with FDA electronic submission systems, incorrect classification of activities, or misunderstanding foreign establishment requirements. Errors in registration data, delayed renewals, or missing U.S. Agent details can increase the risk of warning letters or import alerts.
Proactive regulatory management is key to avoiding these issues and maintaining uninterrupted market access.
How XPRO America Adds Regulatory Value
XPRO America is a professional US FDA Consultancy that supports pharmaceutical companies with end-to-end FDA drug establishment registration and listing services. Their regulatory team assists with initial registrations, annual renewals, drug listing accuracy, and U.S. Agent services for foreign manufacturers. By working with experienced consultants, companies can minimize compliance gaps and stay aligned with evolving FDA expectations.
Organizations looking for dependable regulatory support can initiate assistance by contacting the XPRO America team through support@xproamerica.com, where guidance is tailored to the specific type of drug establishment and business model.
Why Accurate Registration Matters
FDA drug establishment registration is more than a legal obligation. It establishes regulatory credibility, prepares facilities for FDA inspections, and builds confidence with importers and distribution partners. Accurate and up-to-date registration also helps prevent supply chain disruptions and supports long-term growth in the U.S. pharmaceutical market.
By maintaining proper registration and drug listings, drug establishments strengthen compliance readiness and secure a stable pathway to operate successfully within one of the world’s most regulated healthcare markets.
Leave a Reply